When we talk about hcooch ch2 h2o, we’re looking at one of the simplest yet most fascinating reactions in organic chemistry—methyl formate hydrolysis. At its core, this process is part of ester hydrolysis basics, where an ester reacts with water to break down into useful products. Understanding how esters react with water is not just a classroom concept but also a doorway into exploring chemistry fundamentals of esters.
This methyl formate reaction overview gives us a clear picture of the chemical breakdown process, showing how bonds rearrange and new compounds are formed. By studying this reaction, learners can gain an easy organic reaction introduction while also appreciating the broader importance of hydrolysis in industry. From fragrances and solvents to biofuels and green chemistry, these reactions show up in surprising ways in our everyday world.
If you’ve ever wondered “what happens when esters break down” or wanted a beginner guide to ester hydrolysis, this reaction is the perfect example to start with. In this article, we’ll break down the details of ester–water interaction, explain the steps simply, and highlight why this reaction continues to play a vital role in modern science and industry.
Breaking Down the Molecular Components of hcooch ch2 h2o
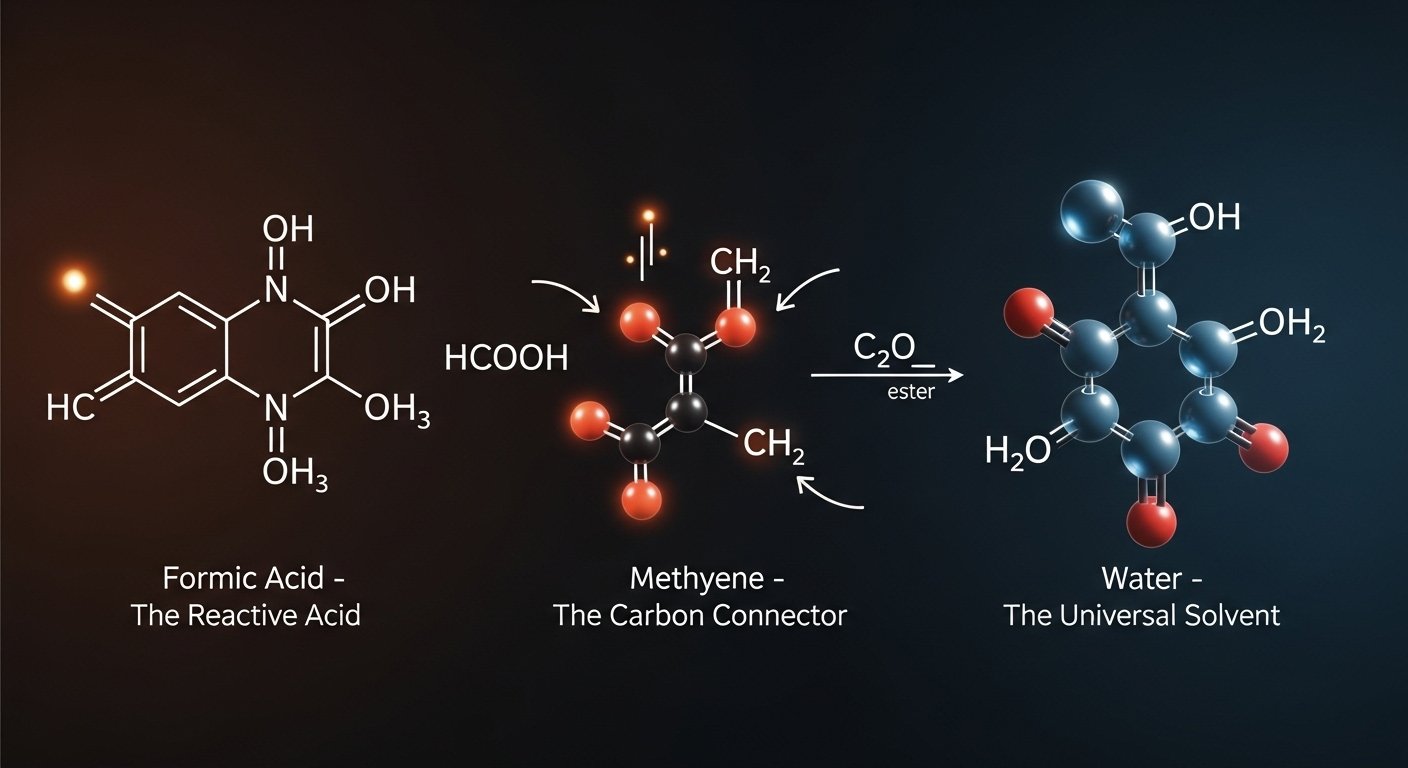
When we talk about hcooch ch2 h2o, we’re really looking at a fascinating combination of three key parts: formic acid (HCOOH), methylene (CH₂), and water (H₂O). Each of these molecules plays a unique role, and together they drive one of the most important reactions in organic chemistry — ester hydrolysis. Let’s break them down one by one.
HCOOH (Formic Acid) – The Reactive Acid
Formic acid is known as the simplest carboxylic acid, but don’t let its simplicity fool you. It has powerful chemical properties that make it a highly reactive organic acid. Found naturally in ant venom and stinging nettles, it’s one of the natural sources of formic acid that scientists first studied centuries ago.
In chemistry labs, formic acid is often used to demonstrate acidity in organic chemistry, showing how hydrogen bonding acids interact with other molecules. Its chemical behavior of carboxylic acids makes it perfect for teaching how formic acid reacts in chemistry, especially in hydrolysis reactions where it acts as a catalyst.
In the United States, its industrial importance is huge. From agricultural uses of formic acid like silage preservation to formic acid derivatives used in leather, rubber, and textiles, this small molecule supports big industries. However, there’s also a toxicology of formic acid side — it’s corrosive and requires careful handling. Still, its role in hydrolysis is what makes it central to the hcooch ch2 h2o reaction.
CH₂ (Methylene) – The Carbon Connector
Now let’s look at methylene (CH₂), often called the carbon connector in organic molecules. The organic methylene group is a common feature in countless compounds, but it’s also one of the most interesting because of its reactivity.
In reactive intermediates chemistry, CH₂ often appears as methylene radicals or other unstable intermediates that exist only briefly before forming new bonds. This is why methylene is reactive — it doesn’t like being alone, so it quickly connects to other atoms.
In everyday chemistry, CH₂ in organic synthesis shows up everywhere: from methylene bridges in molecules that hold structures together to its role in hydrocarbons and methylene-based reactions. Scientists study its stability of methylene compounds and how these organic reaction intermediates drive larger processes. In fact, one of the most fascinating applications of methylene chemistry is its participation in forming esters like methyl formate, making it an essential player in the hcooch ch2 h2o equation.
H₂O (Water) – The Universal Solvent
Finally, we can’t ignore the star of almost every chemical process: water (H₂O). Often called the universal solvent, water’s ability to dissolve a wide range of compounds makes it critical in aqueous chemical reactions. Its unique physical properties of water, such as polarity, allow it to participate in polar solvent chemistry and enable hydrogen bonding in water that stabilizes countless reactions.
When it comes to ester breakdown, water is essential. It is both the solvent for hydrolysis reactions and the active participant, directly attacking the ester bond. That’s why water is the best solvent for this type of chemistry. In fact, without water, the entire process of hcooch ch2 h2o would not occur.
Chemists often highlight the importance of H₂O in hydrolysis, not just as a medium but as a reactant. Its reactivity of water molecules allows it to split bonds, making it vital for water facilitating ester breakdown in both laboratory and industrial settings. Beyond the lab, water’s role in life sciences and organic reactions makes it irreplaceable — proving once again that this humble molecule is central to chemistry and life itself.
How the Hydrolysis of hcooch ch2 h2o Works
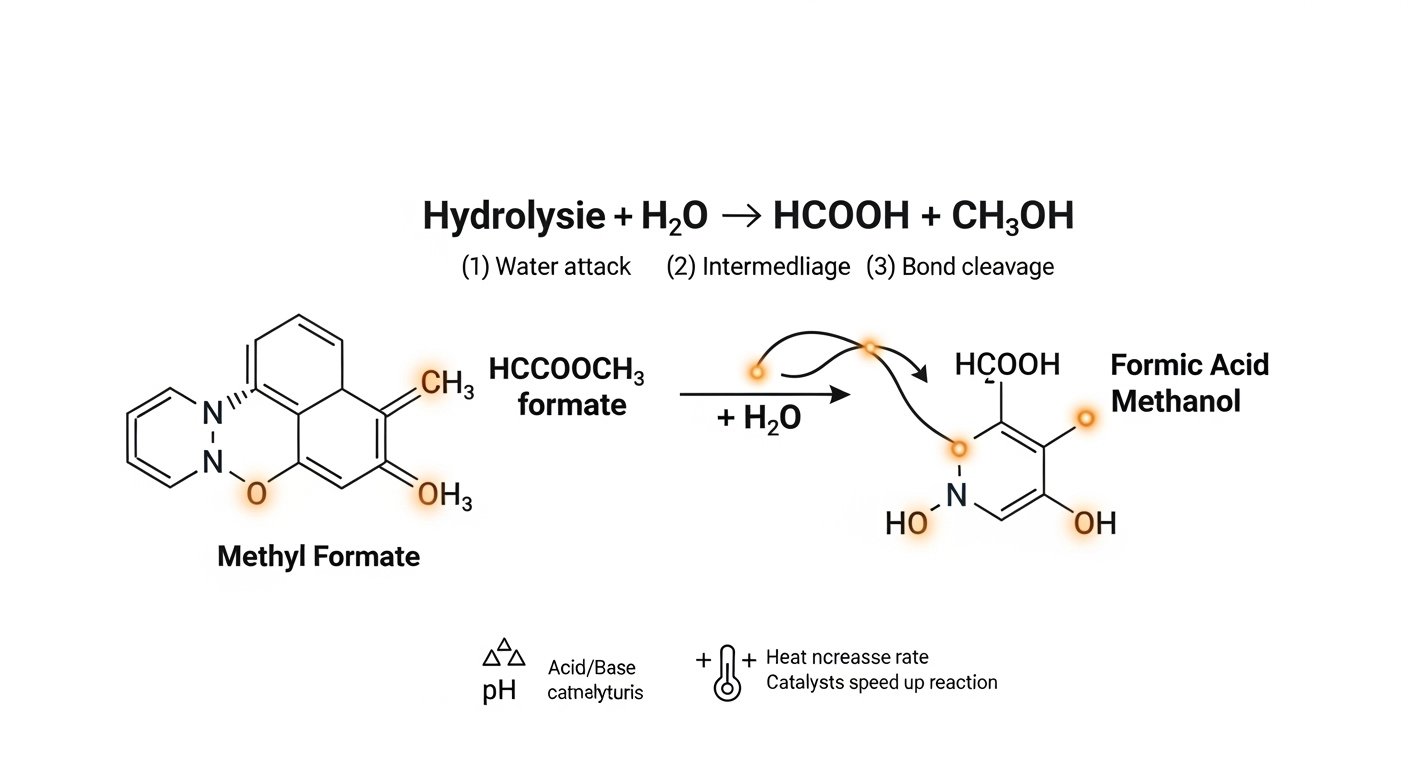
The reaction of hcooch ch2 h2o is a classic example of an ester hydrolysis reaction pathway. In simple terms, hydrolysis means “breaking with water.” Here, methyl formate reacts with water, and the ester bond is split to produce formic acid (HCOOH) and methanol (CH₃OH). This process is widely studied in organic chemistry kinetics because it shows how esters behave under different conditions.
The Chemical Equation and Mechanism
The balanced organic equation for this reaction is:
This equation shows the ester (methyl formate) reacting with water to form two products: formic acid and methanol. It looks simple, but the mechanism of ester breakdown involves several steps.
Here’s a step by step ester hydrolysis process:
Water attacks the ester – In the first step of the reaction pathway for methyl formate, a water molecule acts as a nucleophile and attacks the carbon atom in the ester group.
Intermediate formation – This creates a temporary, unstable structure that rearranges itself.
Bond cleavage – The ester bond breaks (the ester cleavage process) to release formic acid and methanol.
This hydrolysis reaction explained with a reaction mechanism diagram (if included in the article) makes it easy to visualize ester hydrolysis. Students often find it helpful to compare this with cutting a chain link: water is the tool that snaps the bond apart, leaving two free ends.
In some cases, the reaction can be accelerated by acids or bases, leading to catalytic ester hydrolysis. Whether in a lab experiment or industrial setting, the process highlights the beauty of stepwise chemical reactions in organic chemistry.
Factors Influencing Hydrolysis (pH, Temperature, Catalysts)
The speed and outcome of hcooch ch2 h2o hydrolysis depend heavily on the hydrolysis reaction conditions. Chemists study these factors under the lens of chemical kinetics of esters and thermodynamics of ester breakdown.
pH conditions – The reaction can be influenced by acids and bases.
Under acid catalysis, hydrogen ions make the ester bond more vulnerable to attack.
With base catalysis, hydroxide ions accelerate the process even further.
That’s how pH affects ester hydrolysis, making it either slower or faster depending on conditions.
Temperature – Like most chemical processes, higher temperatures increase reaction rates. The temperature impact on reactions is clear: heating provides the energy needed to overcome the activation energy of hydrolysis.
Catalysts – Adding catalysts provides an alternative reaction pathway. This is why catalysts speed up hydrolysis without being consumed in the process. Acid and base catalysts are common catalyst examples in hydrolysis, but enzymes can also play a role in biological systems.
Optimal conditions – The optimal conditions for methyl formate reaction usually involve controlled pH, moderate heating, and in some cases, the use of a catalyst to achieve efficiency while preventing unwanted side reactions.
Think of it like cooking: adjusting the “heat” (temperature), “spice level” (pH), and “cooking tools” (catalysts) can completely change how fast and well the final dish (products) comes out.
Industrial and Practical Applications of hcooch ch2 h2o
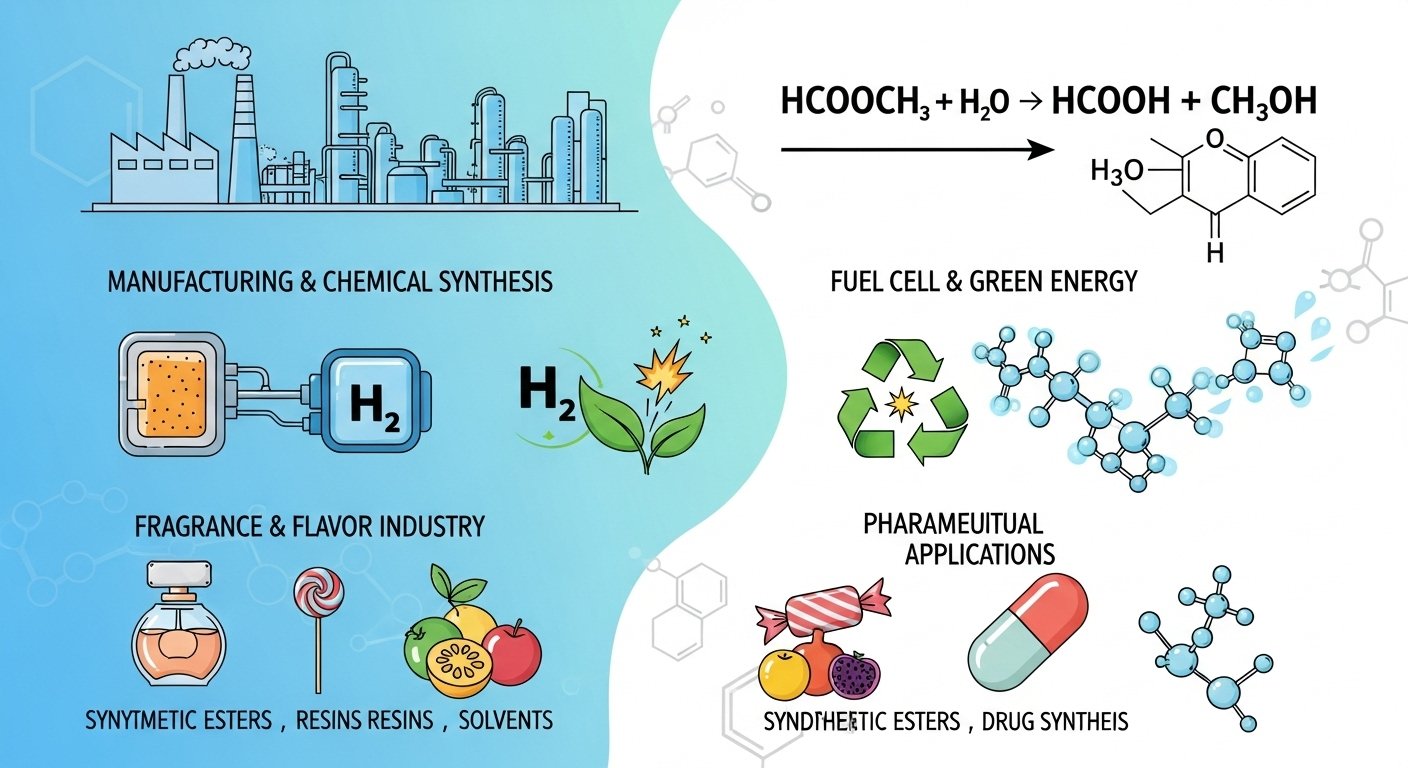
The reaction of hcooch ch2 h2o is not just important in theory—it has a wide range of industrial chemistry processes and everyday applications. From chemical manufacturing to green fuel research, and even perfumes and medicines, its influence stretches across multiple industries. Let’s explore how this reaction shapes the world around us.
Role in Manufacturing & Synthesis of Chemicals
One of the most important roles of hcooch ch2 h2o lies in manufacturing with methyl formate as a chemical feedstock. In simple terms, a feedstock is the “raw ingredient” that industries use to make other chemicals. Methyl formate serves as a starting material in synthetic organic pathways, creating compounds like formic acid, methanol, and formaldehyde.
Production of solvents: Methyl formate is widely used in making solvents that clean, dissolve, or extract chemicals.
Ester intermediates industry: It acts as a bridge in chemical synthesis, making it easier to create new esters.
Bulk chemical production: US chemical plants rely on methyl formate as a cost-effective solution in chemical synthesis pathways.
Raw material for polymers: It is used in certain synthetic processes involving esters, particularly in creating resins and plastics.
If you’ve ever wondered how methyl formate is used in industry, the answer is clear: it helps build countless products we interact with daily, from plastics to coatings. In this sense, the ester role in manufacturing is as essential as bricks in a building—it may not be visible, but nothing stands without it.
Use in Fuel Cell Technology and Green Fuels
Beyond traditional manufacturing, hcooch ch2 h2o is being explored in the race for eco-friendly energy solutions. One of its most exciting applications is in formic acid fuel cells, where formic acid acts as a hydrogen source. This makes it a powerful candidate in renewable energy esters that can replace fossil fuels.
Researchers highlight three key benefits:
Hydrogen carrier compounds – Methyl formate and its derivatives can safely store and release hydrogen.
Low-emission fuels – When used in fuel cells with organic compounds, they produce far fewer emissions compared to gasoline or diesel.
Carbon-neutral fuels – They support sustainable fuel chemistry by reducing greenhouse gases.
In the US, green fuel research is accelerating because industries need renewable alternatives to fossil fuels. With rising concerns over climate change, the role of methyl formate in energy applications is expected to grow. It’s a glimpse into a future where powering your car may not rely on oil, but on smart alternative energy chemicals instead.
Applications in Fragrance, Flavor, and Pharmaceuticals
On the lighter side, hcooch ch2 h2o also contributes to the things that make life enjoyable—scents, tastes, and medicines. Esters like methyl formate are known for their aromatic properties, making them central to the fragrance and flavor applications industry.
Ester aroma compounds – They provide fruity, sweet notes used in perfumes.
Synthetic flavors and fragrances – In the US, food companies use them as flavoring agents in food industry products.
Perfumery esters – Essential in creating complex esters in perfumes and scents.
Pharmaceutical intermediates – Methyl formate supports the role of esters in drug synthesis, enabling the creation of ester-based drugs and other medical ester compounds.
Whether it’s the fragrance of a luxury perfume or the flavor in a candy, chances are you’ve already enjoyed the results of natural vs synthetic esters. And in the medical world, methyl formate in medicine highlights how chemistry can bridge comfort, health, and technology.
Laboratory Context and Experimentation with hcooch ch2 h2o
Understanding the reaction of hcooch ch2 h2o isn’t just about theory—it’s also about practice. In laboratories, ester hydrolysis experiments help both students and researchers visualize chemical reactions in action. These experiments range from easy ester hydrolysis experiments in schools to research-level ester experiments using advanced instruments.
Ester Hydrolysis Experiments (Student Level)
For beginners, teaching hydrolysis to students often starts with simple hands-on activities. A classic high school chemistry ester lab demonstrates how esters break down into alcohols and acids when mixed with water under the right conditions. Teachers use this as a classroom experiment with esters to make complex reactions easier to grasp.
A typical ester hydrolysis lab activity might involve heating methyl formate with dilute acid or base. Students observe changes, note the smell of products, and record results on ester experiment worksheets. These beginner organic lab activities not only reinforce concepts of functional groups and chemical bonds but also build curiosity.
Safety is always key. Student lab safety guidelines—such as wearing goggles, using small sample quantities, and proper waste disposal—make sure the student-level ester hydrolysis demo remains educational without risks. Teachers often add a fun angle by comparing ester smells to real-world fragrances, making organic chemistry education more engaging.
In short, hydrolysis for beginners transforms abstract textbook knowledge into memorable experiences through school chemistry demonstrations that highlight both science and safety.
Advanced Lab Protocols and Techniques
In professional labs, the study of hcooch ch2 h2o goes much deeper. Researchers use advanced organic experiments and professional hydrolysis techniques to control conditions and measure results precisely. These lab procedures for esters often involve pH monitoring, controlled heating, and specialized catalysts to observe controlled ester breakdown.
Modern labs rely on analytical chemistry protocols to study ester hydrolysis. For example:
Spectroscopy methods (like NMR and IR) to identify products.
Chromatography to separate compounds and confirm yields.
Titration to measure acidity changes during the reaction.
Such detailed ester hydrolysis procedures are critical for research-level ester experiments in pharmaceuticals, energy research, and material science. Scientists follow scientific research methods and use modern lab equipment for esters like automated reactors and precision balances.
In lab-scale synthesis, methyl formate hydrolysis provides key intermediates that can be further processed. This highlights why methyl formate testing in labs is not just educational but also vital in industrial R&D.
Ultimately, techniques for accurate hydrolysis allow researchers to explore reaction kinetics, thermodynamics, and catalytic effects—turning a simple student demonstration into a foundation for innovation.
Environmental and Safety Considerations

Working with hcooch ch2 h2o (methyl formate hydrolysis) provides valuable products like formic acid, but it also raises environmental and safety concerns. Both students and professionals need to understand chemical safety protocols and sustainable practices when handling these reactions. By combining safe handling procedures with green chemistry approaches, risks can be minimized while protecting both people and the environment.
Safe Handling of Formic Acid & By-products
One of the main by-products of methyl formate hydrolysis is formic acid, a substance widely used in industries but also classified as corrosive. Many people ask, “is formic acid dangerous?” The answer is yes, if mishandled. Formic acid toxicity can lead to skin burns, eye irritation, and respiratory issues.
To ensure safe handling in US labs, strict OSHA safety guidelines and laboratory safety measures are followed. Key steps include:
Using protective gear for acid handling like gloves, goggles, and lab coats.
Working in well-ventilated areas or fume hoods to reduce inhalation risks.
Storing acids in corrosion-resistant containers to prevent leaks.
Keeping neutralizing agents (like sodium bicarbonate) ready in case of spills.
Industrial chemical facilities also rely on a safety data sheet for formic acid (SDS), which outlines hazards, first aid, and handling protocols. These chemical safety best practices are designed to preventing accidents in labs and ensure compliance with regulatory standards.
In simple terms, treating formic acid and its by-products with respect is crucial. Just like handling fire safely can provide warmth without burns, applying the right protective equipment in chemistry allows safe use of this powerful chemical.
Waste Management and Green Chemistry Approaches
Beyond lab safety, managing by-products responsibly is key. Improper disposal of esters and acids can harm ecosystems, which is why eco-friendly chemical disposal and sustainable lab practices are encouraged worldwide.
When discussing how to dispose chemicals safely, researchers turn to EPA chemical disposal guidelines in the US. These include neutralizing small acid quantities, using designated waste containers, and working with certified chemical disposal services.
Modern labs also explore green chemistry initiatives in USA to reduce their environmental footprint. Examples include:
Using biodegradable solvents instead of harmful organics.
Recycling solvents through distillation in chemistry labs.
Designing eco-friendly ester hydrolysis pathways that minimize toxic by-products.
Applying green synthesis methods to create chemicals with less waste.
Such practices help meet environmental compliance US standards while reducing costs in the long run. In fact, many chemical industries now view sustainability not as an extra step but as a core part of industrial chemical hazards management.
In short, whether it’s waste management in laboratories or environmentally safe chemical reactions, green approaches protect both people and the planet while keeping progress moving forward.
Why hcooch ch2 h2o Chemistry Matters in Modern Science
The hydrolysis of hcooch ch2 h2o (methyl formate with water) may look like a simple textbook reaction, but in reality, it sits at the heart of many modern chemical innovations. From creating essential industrial chemical relevance to shaping sustainable chemical research, this reaction highlights the importance of ester hydrolysis today. Understanding such processes allows scientists to connect basic reactions with global challenges like energy, sustainability, and even chemistry in space exploration.
Chemistry and Sustainability
One of the most exciting aspects of hcooch ch2 h2o is its role in renewable energy chemistry. Hydrolysis reactions help produce low-carbon fuels, create eco-friendly solvents, and even support link between chemistry and sustainability initiatives in industries. For example, converting esters into formic acid and methanol provides building blocks for green fuel technologies.
Think of ester hydrolysis as recycling at the molecular level—it breaks down compounds into smaller, reusable parts. This makes it a critical step in sustainable chemical research, where every atom counts in reducing waste and pollution.
Applications Beyond Earth
The relevance of esters in modern science extends far beyond industrial labs. In fact, scientists studying interstellar organic compounds have found evidence of esters and their hydrolysis products in space. These discoveries provide clues about how life-supporting molecules may form in star-forming regions and on icy comets.
This link between space research involving organic molecules and everyday chemistry shows how the same simple reactions we learn in class could play a role in the origins of life. In this way, hydrolysis becomes a bridge between Earth-based science and the mysteries of the cosmos.
Driving Scientific and Industrial Innovation
In laboratories and industries, the scientific importance of hydrolysis lies in its versatility. The breakdown of esters supports applications of ester hydrolysis in:
Pharmaceutical development – making key intermediates for drugs.
Polymer production – where hydrolysis helps adjust material properties.
Fuel processing – supporting chemistry’s role in renewable energy.
Research and education – serving as a model for teaching cutting-edge organic research.
By offering insights into both theory and practice, ester hydrolysis contributes to the future of modern chemical innovations across multiple fields.
Emerging Research and Future Directions of hcooch ch2 h2o
The study of hcooch ch2 h2o is not just about understanding a simple ester hydrolysis reaction—it’s about unlocking the future of ester hydrolysis research. Scientists are now exploring how advanced tools, new catalysts, and sustainable approaches can transform this classical reaction into a gateway for innovation in both industry and academia. By combining traditional chemistry with computational organic chemistry and AI in chemical research, this area is rapidly becoming a cornerstone of modern scientific exploration.
Computational Tools and AI Integration
One of the most promising areas of growth is the use of computational tools for reaction modeling. Through molecular modeling esters, researchers can predict how reactions like hcooch ch2 h2o will behave under different conditions without needing to run hundreds of physical experiments. This not only saves time and costs but also reduces chemical waste.
Even more exciting is the rise of AI applications in chemistry. Artificial intelligence can now analyze large datasets of reaction conditions, optimize parameters for efficiency, and even suggest next-gen catalysts for hydrolysis that humans might overlook. This integration of digital intelligence with lab science is reshaping the latest discoveries in organic chemistry.
Catalysts and Green Energy Connections
Catalysts have always been the heart of chemical reactions, and novel catalysts for esters are opening up new possibilities. Imagine catalysts that can accelerate green energy reactions while producing minimal by-products. In the case of hcooch ch2 h2o, new catalytic systems are being tested to make the process faster, cleaner, and more energy-efficient.
This innovation connects directly to sustainable fuel chemistry research. By using hydrolysis products like formic acid as hydrogen carriers, scientists are exploring ways to create safer and more efficient fuels. It’s a perfect example of how emerging green chemistry solutions can help reduce reliance on fossil fuels while promoting eco-friendly alternatives.
Trends in Chemical Innovation
Looking ahead, the future of ester hydrolysis will be shaped by three major trends:
Digital Chemistry – More reliance on simulations and AI-driven labs.
Sustainability First – Stronger focus on green energy reactions and waste-free chemistry.
Advanced Materials – Development of smart catalysts and reusable systems for long-term use.
These chemical innovation trends highlight how a simple reaction like hcooch ch2 h2o can evolve into a powerful tool for advancing global science and technology.
Conclusion
The study of hcooch ch2 h2o highlights how a simple ester hydrolysis can unlock deep insights into chemistry, sustainability, and innovation. From understanding the ester breakdown process to applying principles of green chemistry, this reaction shows how science connects theory with real-world applications. Whether in industrial chemical relevance, renewable energy chemistry, or even sustainable lab practices, this process reminds us of chemistry’s powerful role in shaping the future.
By appreciating both the safety aspects and the eco-friendly potential, researchers and learners can see why this small reaction carries such big significance. As modern science moves toward cleaner and smarter solutions, exploring reactions like hcooch ch2 h2o encourages us to think beyond textbooks and into practical impact. It’s not just about bonds breaking; it’s about opening doors to new opportunities in science, industry, and sustainability.
Frequently Asked Questions (FAQs)
What happens during the HCOOCH·CH₂ + H₂O reaction?
The reaction of hcooch ch2 h2o is an ester breakdown process where water reacts with the ester, causing a chemical decomposition reaction. This leads to the products of ester hydrolysis, including formic acid and methanol. In simple terms, it’s the reaction outcome of ester hydrolysis where bonds break, and new molecules form — a classic example of the end products of methyl formate breakdown.
Is CH₂ stable on its own?
The CH₂ group, also called methylene, is not stable under normal conditions. It is considered an unstable intermediate in free radical chemistry and usually exists only for a very short time. Because of its high methylene reactivity, it reacts almost immediately with other molecules. So, if you wonder “can CH₂ exist freely,” the answer is no—it’s a short-lived species that quickly changes.
Where is methyl formate commonly used in real life?
Methyl formate, linked to the hcooch ch2 h2o reaction, is widely used in solvent applications, foam production, and adhesives. It also appears in perfumes and flavors as part of fragrance esters. These industrial uses of methyl formate make it valuable in both factories and consumer products. So when people ask about the everyday uses of methyl formate, it ranges from esters in fragrances and flavors to chemical industries.
Is this reaction eco-friendly?
The hcooch ch2 h2o reaction can be considered eco-friendly when performed under green chemistry principles. Hydrolysis itself is a natural process that often aligns with eco-safe reactions. In research, scientists explore how to apply this in sustainable organic chemistry and renewable chemical processes. In short, while not all ester reactions are harmless, with green chemistry applications, the eco impact of ester reactions can be minimized.
Can this experiment be done safely at home or only in labs?
Performing the hcooch ch2 h2o reaction should be limited to controlled labs. Handling esters like methyl formate involves experiment risks, toxic vapors, and flammable conditions. It is not recommended for DIY chemistry restrictions or home setups. If students wonder, “can students try ester hydrolysis at home,” the answer is no—it requires strict chemistry safety at home and should be left to professionals in safe lab environments.